

Ionization potential (first, electron volts) Radius: covalent (crystal) estimated (angstroms) Magnetic susceptibility (cgs units per mole) Thermal conductivity (watts per metre Kelvin)
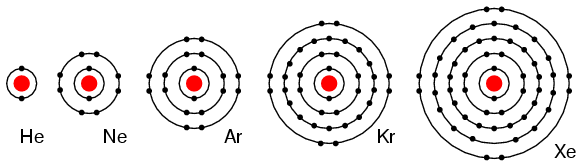
Solubility in water at 20 ☌ (cubic centimetres of gas per 1,000 grams water) **hcp = hexagonal close-packed, fcc = face-centred cubic (cubic close-packed).ĭensity at 0 ☌, 1 atmosphere (grams per litre) Some properties of the noble gases are listed in the table. The nuclei of radium atoms spontaneously decay by emitting energy and particles, helium nuclei ( alpha particles) and radon atoms. Radon usually is isolated as a product of the radioactive decomposition of radium compounds. Most helium is produced commercially from certain natural gas wells. All the noble gases are present in Earth’s atmosphere and, except for helium and radon, their major commercial source is the air, from which they are obtained by liquefaction and fractional distillation. Helium is the most plentiful element in the universe except hydrogen. The abundances of the noble gases decrease as their atomic numbers increase. In chemistry and alchemy, the word noble has long signified the reluctance of metals, such as gold and platinum, to undergo chemical reaction it applies in the same sense to the group of gases covered here. Similarly, use of the term inert has the drawback that it connotes chemical passivity, suggesting that compounds of Group 18 cannot be formed. It is now known, however, that several of these elements are quite abundant on Earth and in the rest of the universe, so the designation rare is misleading. When the members of the group were discovered and identified, they were thought to be exceedingly rare, as well as chemically inert, and therefore were called the rare or inert gases. Their electronic structures and the finding that some of them do indeed form compounds has led to the more appropriate designation, Group 18. They traditionally have been labeled Group 0 in the periodic table because for decades after their discovery it was believed that they could not bond to other atoms that is, that their atoms could not combine with those of other elements to form chemical compounds. The noble gases are colourless, odourless, tasteless, nonflammable gases. The elements are helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), radon (Rn), and oganesson (Og). Noble gas, any of the seven chemical elements that make up Group 18 (VIIIa) of the periodic table.
#Krypton number of valence electrons how to#
COVID-19 Portal While this global health crisis continues to evolve, it can be useful to look to past pandemics to better understand how to respond today.Student Portal Britannica is the ultimate student resource for key school subjects like history, government, literature, and more.From tech to household and wellness products. Britannica Explains In these videos, Britannica explains a variety of topics and answers frequently asked questions.This Time in History In these videos, find out what happened this month (or any month!) in history.#WTFact Videos In #WTFact Britannica shares some of the most bizarre facts we can find.Demystified Videos In Demystified, Britannica has all the answers to your burning questions.Britannica Classics Check out these retro videos from Encyclopedia Britannica’s archives.


 0 kommentar(er)
0 kommentar(er)
